- (305) 252-0963 Ext:100
- 14121 NW 8th St Sunrise FL 33325
Menu
Menu
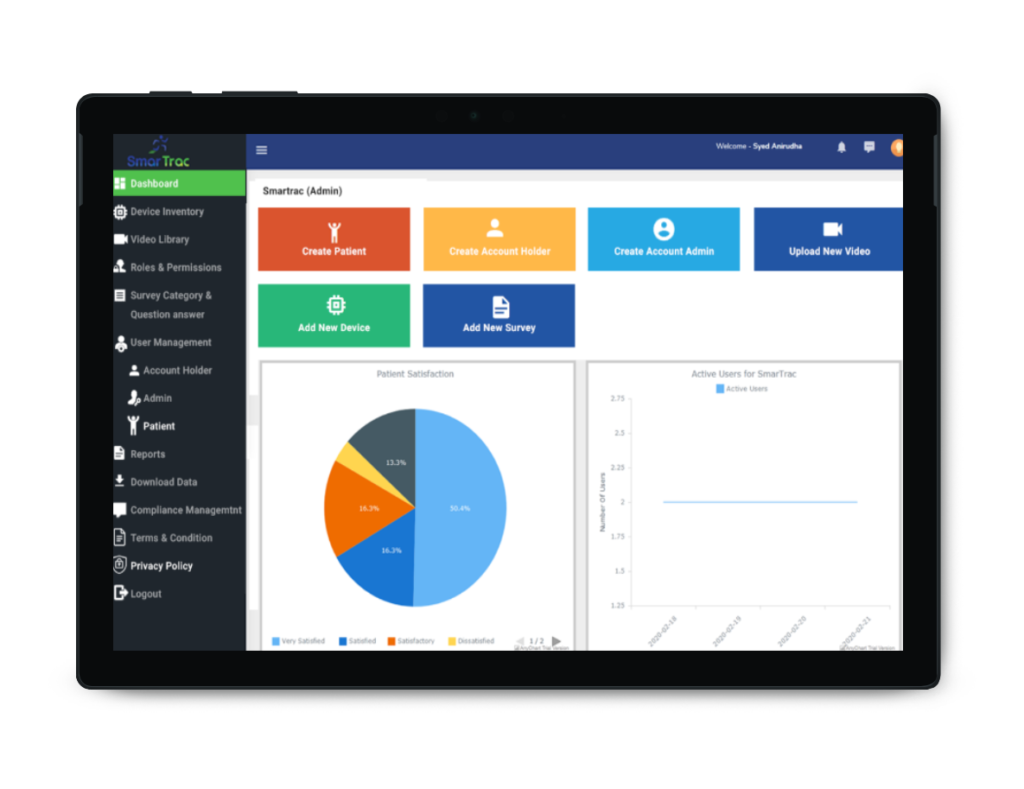
ATTENTION: Physicians and Therapists (PT/OT) can bill for SmarTrac using Remote Therapeutic Monitoring Codes. See corresponding CPT code noted below.
Additionally, DME Providers can bill for SmarTrac using HCPCS code noted below.
The following General Medicine CPT codes for Remote Therapeutic Monitoring are available to Physicians and Therapists (OT & PT). RTM is billed under General Medicine CPT codes, therefore RTM codes are available to Qualified Health Care Practitioners (“QHCPs”) who are both eligible and not eligible to independently order and bill for Evaluation and Management Services. Therefore, these new CPT codes allow physical therapists and occupational therapists to order and bill the RTM codes subject to state scope of practice and supervision requirements.
98975
98977
98980
98981
99453
Remote monitoring of physiologic parameter(s); initial set-up and patient education on use of equipment. CPT 99453 offers reimbursement for the work associated with onboarding a new patient onto a remote monitoring service, setting up the equipment and educating the patient on using the equipment.
99457
Remote physiologic monitoring treatment management services, 20 minutes or more of clinical staff/physician/other qualified healthcare professional time in a calendar month requiring interactive communication with the patient/caregiver during the month. Under 99457, CMS will reimburse for clinical staff time that contributes toward monitoring and interactive
99454
Device(s) supply with daily recording(s) or programmed alert(s) transmission, each 30 days. CPT 99454 offers reimbursement for providing the patient with a remote monitoring device for a 30-day period. Note that 99454 can be billed each 30 days.
99458
Remote physiologic monitoring treatment management services, 20 minutes or more of clinical staff/physician/other qualified healthcare professional time in a calendar month requiring interactive communication with the patient/caregiver during the month. Under 99457, CMS will reimburse for clinical staff time that contributes toward monitoring and interactive
The FDA considers a product to be a device, and subject to FDA regulation, if it meets the definition of a medical device per Section 201(h) of the Food, Drug, and Cosmetic Act.
Per Section 201(h) of the Food, Drug, and Cosmetic Act, a device is:
An instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is:
Sign up to receive product updates
Join our email list to get product updates.
Feel free to contact us. We are more than happy to answer all of your questions about SmarTrac.
Feel free to contact us. We are more than happy to answer all of your questions about SmarTrac.
Tel: (305) 252-0963 Ext 100